
Radiosensitization by gold nanoparticles: effective at megavoltage energies and potential role of oxidative stress
Introduction
The multidisciplinary field of nanotechnology is defined by the United States Nanotechnology Initiative as ‘the understanding and control of matter at dimensions of roughly 1-100 nanometers where unique phenomena enable novel applications’. As nanomaterials are similar in size to many biological molecules, they have much potential for a wide range of applications in the biomedical field. In particular, gold nanoparticles (GNPs) have demonstrated significant potential as diagnostic imaging agents, drug delivery platforms and radiation sensitizers. These applications are due to the attractive physico-chemical characteristics of GNPs which make them biologically inert and easy to functionalize with drugs or moieties for active targeting.
In this review, we consider the physical properties of GNPs which make them widely utilizable in the field of radiation research as image contrast agents, drug delivery vehicles and radiation sensitizers. In particular, we focus on the growing amount of preclinical evidence demonstrating the radiosensitizing properties of GNPs which has shown that biological effects cannot be accurately predicted on the basis of GNP concentration and beam energy and suggest oxidative stress as a central mechanism in mediating response.
Physical properties of GNPs for applications in radiation research
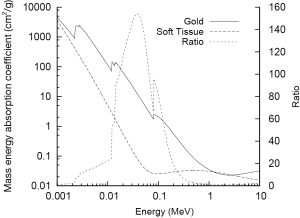
GNPs are widely applicable in radiation research largely due to the high atomic number (Z) of gold (Z=79) which results in significant differences in mass energy absorption properties compared to soft tissue. The mass energy absorption coefficients of both gold and soft tissue are shown as a function of photon energy in Figure 1. The high Z of gold and corresponding large number of bound electrons gives it a very high mass energy absorption coefficient for X-rays which is significantly larger than that of soft tissue over a wide energy range and consequently enables GNPs to act as effective X-ray contrast agents.
In addition to preferential mass energy absorption, GNPs are attractive agents in the field of radiation research as they are relatively easy and inexpensive to synthesize, they can be synthesized in a range of sizes (2-500 nm) and different shapes by altering synthesis conditions and have highly reactive surfaces making them easily modified by conjugation to drugs or active targeting moieties. In the absence of active targeting, GNPs have an inherent ability to passively accumulate in tumor cells through the abnormal vasculature of solid tumours via fenestrated vessels allowing permeation of GNPs into the tumour where they may be retained due to poor lymphatic function, a phenomena known as the enhanced permeability and retention effect (EPR) (2).
GNPs as image contrast agents
Diagnostic imaging is a valuable tool in providing information on the size, shape and anatomical location of tissues within the body that can be significantly improved with the use of contrast agents. Typically, diagnostic X-ray imaging is performed at energies less than 200 keV. At these energies, photon absorption occurs through two main processes (shown schematically in Figure 2); the Compton effect and the photoelectric effect, with the Compton effect being the dominant process for interactions with soft tissue. In this process, an incident photon is scattered by a bound electron which loses energy to eject the electron from the atom. This process is only weakly dependent on both photon energy and atomic number and consequently is the dominant process in materials of low Z such as soft tissue.
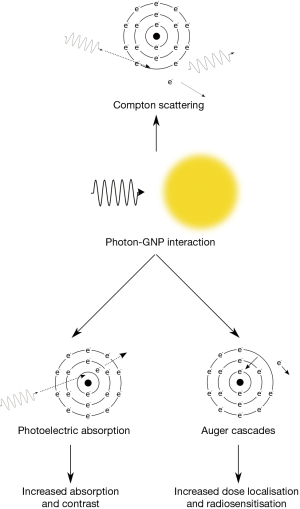
The photoelectric effect is the competing process whereby incident photons are wholly absorbed by bound electrons which are then ejected from the atom rather being scattered. As a result of this complete absorption, there is a significant dependence on both photon energy and atomic number with significant jumps in absorption occurring when photons possess just enough energy to eject an electron from a target atom (as can be seen in Figure 1). Due to the high Z of gold there are several of these characteristic absorption edges in the low keV energy range (from 1 to 100 keV) which leads to much stronger absorption of X-rays in this energy range. This increased absorption translates into significant contrast with soft tissue at kilovoltage imaging energies where the majority of photon interactions with GNPs occur through the photoelectric effect. At 100 keV, gold will provide around 2.7 times greater contrast per unit weight compared to iodine (Au =5.16 cm2•g-1; I =1.94 cm2•g-1; soft tissue =0.169 cm2•g-1; bone =0.186 cm2•g-1) (1).
The improvements in image contrast offered by GNPs were first demonstrated by Hainfeld and colleagues at energies of 80-100 keV using 1.9 nm particles systemically delivered to mice bearing subcutaneous tumours (3). Compared to iodine based contrast agents, this study showed improved delineation of blood capillaries, reduced bone interference and imaging times with no toxicity at 11 and 30 days after injection.
Further enhancement of image contrast may be achieved by improving the target specificity via active targeting of GNPs to specific cell surface receptors. This approach was demonstrated by Hainfeld and colleagues using anti-human epidermal growth factor receptor 2 (Her2) antibody (Trastuzumab) to target GNPs to Her2 positive BT-474 tumors (4). In addition, Reuveni et al., demonstrated significantly enhanced imaging using anti-epidermal growth factor receptor (EGFR) antibody (Cetuximab) functionalized particles targeting EGFR positive A431 head and neck tumors (5). Another approach by Chandra et al., has involved bombesin functionalized GNPs targeting the gastrin releasing peptide receptor (GRPT) in mice bearing PC-3 prostate tumors (6). These approaches are likely not only to lead to improvements in image contrast but could potentially improve the detection of tumors based on tumor specific biomarkers and also allow a reduction in imaging dose which is of importance for scenarios where repeated imaging is required.
GNPs as platforms for anticancer drug delivery
GNPs have a number of properties which make them attractive vehicles for drug delivery; these include low toxicity, high surface area, biocompatibility and ease of fabrication into ranges of size and shape. An additional important property of GNPs is the ease with which they can be functionalized through established gold-thiol chemistry on the nanoparticle surface (7). Drugs may be linked to GNPs using a number of strategies including covalent and non-covalent attachment, encapsulation and electrostatic adsorption (8). These approaches have the potential to improve the biodistribution and pharmacokinetics of many low molecular weight compounds used therapeutically including anticancer drugs which are highly toxic and often have poor bioavailability due to their hydrophobic nature (9). Furthermore, adverse toxic effects in normal tissue may be minimised by selective targeting to tumor cells through functionalization with small molecule ligands, polymers or biological moieties including peptides, antibodies or nucleic acid sequences (10).
Platinum based compounds have been used for over 40 years to treat a range of human cancers (11). A number of approaches have been used to functionalize platinum complexes to GNPs which have shown increased efficacy in comparison to the free compound. Dhar et al., demonstrated significantly increased cytotoxicity in vitro using amine functionalized polyvalent oligonucleotide GNPs as delivery vehicles for cisplatin and compared to cisplatin alone (12). In a recent study by Comenge et al., GNPs were shown to reduce the toxicity of cisplatin without loss of therapeutic efficacy and improved biodistribution in a human lung cancer model in vivo (13). Other approaches have included folic acid conjugation of GNPs bound to Cisplatin for targeted delivery in ovarian cancer (14). A study by Brown et al., showed improved uptake and activity using GNPs functionalised with the active component of oxaliplatin [Pt(Dach)] in range of colon cancer models in vitro (15).
The application of GNPs for improved delivery of anticancer drugs has not been limited exclusively to platinum based compounds. Several other small molecule therapeutics have been successfully conjugated to GNPs using different chemistries including paclitaxel (16) and doxorubicin (17). Zhang et al., demonstrated increased solubility and efficacy of paclitaxel through conjugation to 13 nm GNPs (18). Wang et al., showed enhance cytotoxicity and apoptosis with functionalization of doxorubicin to 30 nm citrate capped GNPs in MCF-7 breast cancer cells (17).
Several attempts to improve drug efficacy with GNPs have involved active targeting to specific cell surface receptor. Dreaden et al., observed a 2.7 fold increase in the potency of tamoxifen when conjugated to GNPs to selectively target estrogen receptor positive tumour cells in vitro (19). Further examples have been provided by Patra et al., who demonstrated significant tumour growth inhibition following targeted delivery of 5 nm GNP bound Gemcitabine to the EGFR using Cetuximab in an orthotopic pancreatic cancer model (20). Paclitaxel functionalised GNPs to biotin expressing tumor cells via the biotin receptor has shown significant activity in a range of tumour cell types (21).
The conjugation of drugs and active targeting agents to the GNPs give rise to the potential for multimodal agents in which improvements in bioavailability and systemic toxicity of anticancer compounds can be achieved along with the ability to further enhance other treatment options such as radiotherapy. An example of where this approach may be particularly advantageous in the clinic is in the treatment of malignant glioma. Malignant glioma is one of the most common primary cerebrospinal tumors. The current standard of care for patients with high grade glioma is temozolomide with adjuvant radiotherapy which results in a median survival of 11-33 months after diagnosis and 7 months following recurrence (22,23). Orza et al., recently demonstrated a two fold increase in glioblastoma cancer stem cell kill in vitro using Temozolomide conjugated to the surface of L-aspartate treated GNPs of 55 nm triangular nanoparticles compared to Temozolomide alone (24). Although the current study did not investigate the effects when combined with radiation, it can be envisaged that such an approach could offer improved efficacy of combined chemo-radiation strategies leading to improved patient outcome.
GNPs as radiation sensitizers
The application of GNPs as radiation sensitizers is based on their ability to increase dose deposition in the target volume due to differences in mass energy absorption coefficient when compared to soft tissue as shown in Figure 1. Observations of dose enhancement occurring from high Z materials were first observed in patients with reconstructive metal implants receiving radiotherapy for mandibular and head and neck cancers (25-27). Several approaches have been made to improve photoelectric absorption in the tumor cells using gold in different forms including foil (28) and microspheres (29) and whilst they provide a rationale for the application gold as a radiosensitizer their application is limited by delivery to the tumour target. GNPs are a more attractive approach due to their ability to passively accumulate in tumor cells through the EPR and their small size allows for more uniform uptake and distribution of dose enhancement within the cellular environment.
Physical processes
Similar to the mechanisms of energy absorption of gold when used for imaging purposes, for scenarios where radiosensitization is desirable, increased energy deposition is driven by differences in the absorption characteristics of the high Z gold atoms compared to the low Z soft tissue. At lower keV energies (such as those used in the majority of preclinical studies), the strong photoelectric absorption leads to dramatic increases in the absorbed dose with a concentration of 1% by mass gold leading to approximately a doubling of the dose delivered (Table 1). Despite the magnitude of these effects, this approach is limited in clinical application to superficial tumors due to the low penetration of keV energy photons with half lengths in the order of 1 cm.
Full table
In most clinical scenarios, radiotherapy is delivered using megavoltage photons with energies typically ranging from 1 to 15 MeV. At these energies, photon absorption in both gold and soft tissue is dominated by the Compton effect. Due to its relatively weak dependence on atomic number, this leads to greatly reduced contrast with GNPs typically only increasing the total dose deposited by a few percent. However, it is important to note that although the total dose deposition is largely unchanged, ionising events in GNPs tend to lead to different distributions of energy deposition compared to those in soft tissue. In particular, high-energy Compton scatter events in soft tissue tend to distribute their energy over a wide range as both the Compton electron and scattered photon high energies and long ranges.
In contrast, ionisation events in GNPs have the potential to deposit localised hot-spots of dose due to the production of Auger electrons. Auger electrons are produced when an ionisation occurs in an inner electron orbital leaving a vacancy which can be filled by an electron falling from a higher shell into the lower shell. This process releases energy typically through further electron emission and as each emitted electron leads to the creation of an additional vacancy, this process can cascade leading to the emission of a large number of secondary electrons; possibly as many as 20 in the case of an inner-shell ionisation in gold. Auger electrons are typically very low energy (<5 keV) and short range, meaning they deposit very high doses in the vicinity of the nanoparticle (ranges of ~tens of nm). As a result, this leads to very high dose levels in the vicinity of an ionised GNP which may contribute to their increased biological effectiveness.
Preclinical evaluation of GNPs as radiosenstizers
The first evidence of GNPs as radiosensitizers was demonstrated by Hainfeld and colleagues in mice bearing subcutaneous EMT-6 mouse mammary carcinomas using 1.9 nm thiol coated Aurovist™ (Nanoprobes Inc) in combination with 250 kVp X-rays (42). Following systemic delivery of particles at a maximum dose of 2.7 g Au/kg-1, complete tumour regression was observed coupled with an increase in one survival from 20% for X-ray irradiated to 86% for animals irradiated in combination with GNPs. This study, for the first time demonstrated the potential of GNPs to radiosensitize in vivo but offered no potential underlying mechanism beyond physical dose enhancement predicted by GNP from mass energy attenuation and GNP concentration. Subsequently, a number of in vitro and in vivo studies aimed to further validate GNPs as radiosensitizers in combination with ionising radiation using a range of different radiation sources summarized in Tables 1,2.

Full table
The vast majority of studies used GNPs in combination with external beam radiation at keV and MV photon energies, however, emerging evidence has also suggested potential radiosensitizing effects of GNPs for other radiotherapy approaches including proton therapy and brachytherapy. Kim et al., showed significant reduction in tumor volume and regression of the CT26 mouse tumour model following systemic delivery of 2 and 13 nm 300 mg Au/kg GNPs irradiated in the spread out Bragg peak (SOBP) of a 41.7 MeV proton beam (46). Similar effects have been reported by the same group, demonstrating a 37-62% increase in complete tumour regression along with a significant increase in one year survival compared to proton only animals irradiated animals (47). These effects were attributed to dose enhancement potentially mediated enhanced ROS production.
Theoretical reports have suggested GNP radiosensitization when they are used in combination with continuous low dose rate brachytherapy (48). This has been supported experimentally by Ngwa et al., who observed a sensitizer enhancement ratio of 1.3 based on residual γ-H2AX in cell cultures exposed to continuous low dose rate brachytherapy using 125I seeds (49).
Considering the significant amount of preclinical experimental evidence demonstrating the radiosensitizing effects of GNPs in vitro and in vivo, it is difficult to draw overall general conclusions due to differences in the properties of the particles investigated such as sizes, charge and surface functionalization and the tumor models used. Whilst these physical characteristic have little impact on dose enhancement, differences in size and/or surface coating may significantly alter the level of intracellular oxidative stress induced by the particles and therefore modify their overall radiosensitizing effect. Additional complexity also arises when considering differences in source energy and potentially radiation quality as other radiation types are investigated.
Dose enhancement does not predict biological effect
Despite the significant variability in experimental parameters in preclinical studies, it is apparent that the observed biological effects of GNP radiosensitization cannot be accurately predicted. Based on the ratio of mass energy absorption coefficients of gold and soft tissue, the addition of 1% of gold by mass to the tumor would result in an approximate doubling of the amount of energy deposited by a kilovoltage X-ray source. In agreement with these simple predictions, several theoretical reports using a range of different GNPs, radiation sources and target geometries (50-54) has suggested dose enhancement factors which do not agree with observed radiobiological effects in most cases. The lack of agreement between predicted and observed biological effects is illustrated in Figure 3. Here, the increase in physical X-ray dose predicted from the addition of the GNPs (based on gold concentration and source energies) is compared to the experimentally observed sensitizer enhancement ratio in biological assays for the studies described in Table 1.
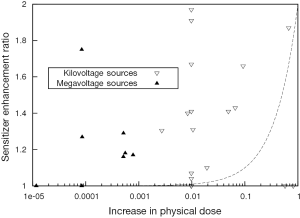
The increase in physical dose quoted refers to the difference between the dose deposited in the cell/GNP system compared to the dose deposited to the cells alone from the same source. The change in aggregate mass energy attenuation coefficients were calculated based on a simple combined model of those of gold and soft tissue, according to the GNP concentrations used in the study. The deposited dose was then calculated by integrating representative X-ray spectra for the sources used in each study over the resulting attenuation data. Finally, the dose enhancement was calculated as the ratio of the dose deposited in the presence of gold, to that in the absence of gold.
As can be seen in Figure 1, in the keV energy range, gold’s mass energy absorption coefficient is roughly 100 to 150 times that of soft tissue. Thus, based on this simple model, it is typically estimated that significant (e.g., greater than 10%) increases in physical dose deposition can be obtained by delivering 0.1-1% by mass GNPs. However, these data show clear radiosensitisations at gold concentrations much lower than this. In addition, significant levels of radiosensitization have been shown in several reports using MV X-rays and electrons. Taken together, these data suggest that a significant component of the overall radiosensitizing effect of GNPs is due to biologically driven processes and cannot be predicted on the basis of GNP concentration and beam energy.
These effects are particularly apparent in the case of studies of megavoltage radiation where gold offers a minimal increase in physical dose but a significant level of radiosensitization (Table 1, Figure 3). These effects must therefore be dependent on factors other than strong photoelectric absorption such as the increased Auger emission occurring from ionisations in gold as discussed previously or additional oxidative stress induced by the presence of the GNPs.
Experimental evidence supporting this concept was provided in a study from our laboratory by Jain et al., (36) who showed GNPs to sensitize to the radiomimetic agent bleomycin to a similar level to that observed for kilovoltage photons, with sensitizer enhancement ratios of 1.38 and 1.41 respectively. Considering that bleomycin is known to induce oxidative stress and DNA damage through the production of reactive oxygen species (ROS) including hydroxyl radicals, superoxide and hydrogen peroxide (55,56), these observations not only validate the concept that GNP radiosensitization is driven primarily through biological processes but strongly suggest a central role of oxidative stress. This is further reinforced by the observations that bleomycin has been shown to increase ROS levels leading to downstream caspase activation and mitochondrial dysfunction in a lung cell model (57).
Oxidative stress is a central mechanism of GNP radiosensitization
Oxidative stress is one of the main mechanisms underlying cellular response to ionising radiation. Free radicals produced from the radiolysis of water can cause direct damage to cellular DNA or may lead to oxidation of lipids and proteins to initiate apoptotic and necrotic cell death process through the mitochondria (58). Elevated level of ROS has been reported for GNPs of various sizes, shapes and surface functionalization to in vitro (59-62). Pan et al., showed a time dependent increase in ROS levels leading to necrotic cell death for 1.4 nm GNPs but not 15 nm particles of the same surface chemistry (60). GNP size was also identified as a key factor in cytotoxicity by Zhang et al., (63) who observed increased levels of apoptosis and necrosis for 4.8 nm PEG-coated GNPs compared to larger PEG-coat GNPs. Similar oxidative effects have also been observed using iron-core gold coated nanoparticles suggesting induction of oxidative is a specific response to gold (64). In addition, the hydrophobicity of the surface coating has also been shown to be a factor in the induction of ROS, with the most hydrophobic coatings being shown to induce the greatest levels of ROS (61).
The precise mechanism by which GNPs induce oxidative stress remains to be fully determined. This could potentially be due direct chemical interaction of the GNPs with cellular molecules such as glutathione, or an effect on biological processes induced by GNP uptake such as stress of the endoplasmic reticulum (ER). ER stress has been validated as a potential mechanism of oxidative stress being shown to occur as an early stage cellular response to GNPs preceding the production of ROS and subsequent cytochrome C release due to mitochondrial damage ultimately resulting in apoptotic and necrotic cell death.
Although accumulating evidence supports oxidative stress as a central mechanism of GNP radiosensitization, a small number of reports have demonstrated GNPs as anti-oxidants. Tournebize et al., demonstrated surface functionalization to have an important role in redox homeostasis showing dihydroplipoic acid functionalized particles to have little effect whilst citrate stabilized GNPs to reduce intracellular glutathione levels by around 20% with no increase in ROS production, apoptosis or upregulation of genes related to oxidative stress (65). In addition, cerium oxide supported GNPs have been shown to exhibit strong antioxidant properties at levels greater than glutathione following rotenone induced ROS production (66).
Considering the growing number of reports from the field focussing on cellular interactions on the nanoscale, relatively few studies have detailed interactions of GNPs in combination with radiation. Using plasmid DNA as a model system to investigate radiation responses, GNPs have been shown to generate hydroxyl radicals in an energy and concentration dependent manner (67,68). Similar effects have been observed in vitro by Geng et al., who showed high levels of intercellular ROS leading to higher levels of oxidative stress and apoptosis in GNP exposed ovarian cancer cells at both kilovoltage and megavoltage X-rays (35).
The underlying mechanisms mediating cellular responses to GNPs in combination with radiation remain to be fully determined. It is evident that biological processes including oxidative stress play an important role in the radiosensitizing effect of GNPs which may occur indirectly by the activation of biological processes which predispose cells to the detrimental effects of radiation.
Perspective
The expansion of nanotechnology into biomedicine presents many exciting opportunities which have much potential to translate into improved strategies for the detection, diagnosis and treatment of cancer. GNPs are intriguing agents, especially for applications in radiation research where their unique physical and chemical properties can be exploited for combined approaches as image contrast agents, drug delivery vehicles and radiation sensitizers.
Despite the significant promise of GNPs for these applications from an ever increasing number of preclinical studies, few GNP based compounds have progressed to early phase clinical trial. An agent which has reached phase 2 clinical trials is CYT-6091 (Aurimmune, CytImmune Sciences, Clinical Trial Numbers, NCT00356980 and NCT00436410). CYT-6091 is a 27 nm GNP functionalized with human recombinant tumour necrosis factor alpha (TNF-α) and PEG which has been trialled for the treatment of a range of advanced cancer types and shown improved tolerance with patients 20 times normal dose of TNF-α (69). Currently no phase I trials have been performed with GNPs as radiation sensitizers. However, a phase I non-randomized trial is active for the treatment of soft tissue sarcoma using standard a 2 Gy radiation schedule in combination with intratumour injection of NBTXR3 prior to surgery (hafnium oxide nanoparticles, Nanobiotix, Clinical Trial Number NCT01433068).
In order to fully translate the significant preclinical benefits of GNPs into clinical practice, further understanding of the precise mechanisms by which their effects are mediated is necessary to drive their use towards early phase clinical trials. This is not a trivial task for two reasons; firstly, preclinical studies have been performed using a wide range of particles of different size and surface coating irradiated with sources of different energy preventing meaningful comparison of radiobiological response. Secondly, biological effects cannot accurately be predicted form physical dose enhancement parameters. Finally, a number of important in vivo parameters which are likely to impact on clinical outcome remain to be fully determined including the radiation induced damage to the tumor vasculature during fractioned treatment which may increase bioavailability at the tumor site as discussed recently by Joh et al. (70). Despite these challenges, GNPs are highly applicable to the field of radiation research and have demonstrated as viable agents for image contrast, drug delivery and biological radiation sensitizers.
Acknowledgments
The authors are grateful to the EU Marie-Curie Training Network ARGENT (608163) for supporting their work.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rao V. L. Papineni, Pataje G.S. Prasanna, Mansoor M. Ahmed) for the series “Nanotechnology in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.08.03). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hubbell JH, Seltzer SM. Tables of X-ray Mass Attenuation coefficients and mass energy-absorption coefficients from 1 keV to 20MeV for Elements Z=1-92 and 48 Additional Substances of Dosimetric Interest, National Institute of Standards and Technology, US Department of Commerce, Gaithersburg, MD 20899, 1996.
- Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 2000;65:271-84. [PubMed]
- Hainfeld JF, Slatkin DN, Focella TM, et al. Gold nanoparticles: a new X-ray contrast agent. Brit J Radiol 2006;79:248-53. [PubMed]
- Hainfeld JF, O’Connor MJ, Dilmanian FA, et al. Micro-CT enables microlocalisation and quantification of Her2-targeted gold nanoparticles within tumour regions. Brit J Radiol 2011;84:526-33. [PubMed]
- Reuveni T, Motiei M, Romman Z, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int J Nanomedicine 2011;6:2859-64. [PubMed]
- Chanda N, Kattumuri V, Shukla R, et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc Natl Acad Sci U S A 2010;107:8760-5. [PubMed]
- Jadzinsky PD, Calero G, Ackerson CJ, et al. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 A resolution. Science 2007;318:430-3. [PubMed]
- Vigderman L, Zubarev ER. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv Drug Deliv Rev 2013;65:663-76. [PubMed]
- Langer R. Drug delivery and targetting. Nature 1998;392:5-10. [PubMed]
- Mout R, Moyano FD, Rana S, et al. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev 2012;41:2539-44. [PubMed]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573-84. [PubMed]
- Dhar S, Daniel WL, Giljohann DA, et al. J Am Chem Soc 2009;131:14652-3. [PubMed]
- Comenge J, Sotelo C, Romero F, et al. Detoxifying antitumoral drugs via nanoconjugation: the case of gold nanoparticles and cisplatin. PLoS One 2012;7:e47562 [PubMed]
- Patra CR, Bhattacharya R, Mukhopadhyay D, et al. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv Drug Deliv Rev 2010;62:346-61. [PubMed]
- Brown SD, Nativo P, Smith JA, et al. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc 2010;132:4678-84. [PubMed]
- Gibson JD, Khanal BP, Zubarev ER. Paclitaxel-functionalized gold nanoparticles. J Am Chem Soc 2007;129:11653-61. [PubMed]
- Wang F, Wang YC, Dou S, et al. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011;5:3679-92. [PubMed]
- Zhang XQ, Xu X, Lam R, et al. Strategy for increasing drug solubility and efficacy through covalent attachment to polyvalent DNA-nanoparticle conjugates. ACS Nano 2011;5:6962-70. [PubMed]
- Dreaden EC, Mwakwari SC, Sodji QH, et al. Tamoxifen-poly(ethylene glycol)-Thiol gold nanoparticle conjugates: Enhanced potencu and selective delivery for breast cancer treatment. Bioconjug Chem 2009;20:2247-53. [PubMed]
- Patra CR, Bhattacharya R, Wang E, et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res 2008;68:1970-8. [PubMed]
- Heo DN, Yang DH, Moon HJ, et al. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 2012;33:856-66. [PubMed]
- Henke G, Paulsen F, Steinbach JP, et al. Hypofractionated reirradiation for recurrent malignant glioma. Strahlenther Onkol 2009;185:113-9. [PubMed]
- Buie L, Valgus J. Current treatment options for the management of malignant glioma. Hematol Oncol Pharm 2012;5:57-63.
- Orza A, Soriţău O, Tomuleasa C, et al. Reversing chemoresistance of malignant glioma stem cells using gold nanoparticles. Int J Nanomedicine 2013;8:689-702. [PubMed]
- Castillo MH, Button TM, Doerr R, et al. Effects of radiotherapy on mandibular reconstruction plates. Am J Surg 1988;156:261-3. [PubMed]
- Allal AS, Richter M, Russo M, et al. Dose variation at bone/titanium interfaces using titanium hollow screw osseointegrating reconstruction plates. Int J Radiat Oncol Biol Phys 1998;40:215-9. [PubMed]
- Niroomand-Rad A, Razavi R, Thobejane S, et al. Radiation dose perturbation at tissue-titanium dental interfaces in head and neck cancer patients. Int J Radiat Oncol Biol Phys 1996;34:475-80. [PubMed]
- Regulla D, Schmid E, Friedland W, et al. Enhanced values of the RBE and H ratio for cytogenetic effects induced by secondary electrons from an X-irradiated gold gurface. Radiat Res 2002;158:505-15. [PubMed]
- Herold DM, Das IJ, Stobbe CC, et al. Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int J Radiat Biol 2000;76:1357-64.30.
- Butterworth KT, Coulter JA, Jain S, et al. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: potential application for cancer therapy. Nanotechnology 2010;21:295101 [PubMed]
- Chang MY, Shiau AL, Chen YH, et al. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci 2008;99:1479-84. [PubMed]
- Chien C, Wang C, Hua T, et al. Synchrotron Radiation Instr 2006:1908-11.
- Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res 2010;173:719-28. [PubMed]
- Coulter JA, Jain S, Butteworth KT, et al. Cell dependent uptake and cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomedicine 2012;7:2673-85. [PubMed]
- Geng F, Song K, Xing JZ, et al. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology 2011;22:285101 [PubMed]
- Jain S, Coulter JA, Hounsell AR, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys 2011;79:531-9. [PubMed]
- Kong T, Zeng J, Wang X, et al. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small 2008;4:1537-43. [PubMed]
- Liu CJ, Wang CH, Chen ST, et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys Med Biol 2010;55:931-45. [PubMed]
- Rahman WN, Bishara N, Ackerly T, et al. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomedicine 2009;5:136-42. [PubMed]
- Roa W, Zhang X, Guo L, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology 2009;20:375101 [PubMed]
- Zhang X, Xing JZ, Chen J, et al. Enhanced radiation sensitivity in prostate cancer by gold-nanoparticles. Clin Invest Med 2008;31:E160-7. [PubMed]
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 2004;49:N309-15.
- Hainfeld JF, Dilmanian FA, Zhong Z, et al. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys Med Biol 2010;55:3045-59. [PubMed]
- Hainfeld JF, Smilowitz HM, O’Connor MJ, et al. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine (Lond) 2012. [Epub ahead of print].
- Hebert EM, Debouttiere PJ, Hunting DJ, et al. MRI detectable gadolinium-coated gold nanoparticles for radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:S715-6.
- Kim JK, Seo SJ, Kim KH, et al. Therapeutic application of metallic nanoparticles combined with particle-induced x-ray emission effect. Nanotechnology 2010;21:425102 [PubMed]
- Kim JK, Seo SJ, Kim HT, et al. Enhanced proton treatment in mouse tumors through proton irradiated nanoradiator effects on metallic nanoparticles. Phys Med Biol 2012;57:8309-23. [PubMed]
- Bahreyni Toossi MT, Ghorbani M, Mehrpouyan M, et al. A Monte Carlo study on tissue dose enhancement in brachytherapy: a comparison between gadolinium and gold nanoparticles. Australas Phys Eng Sci Med 2012;35:177-85. [PubMed]
- Ngwa W, Korideck H, Kassis AI, et al. In vitro radiosensitization by gold nanoparticles during continuous low-dose-rate gamma irradiation with I-125 brachytherapy seeds. Nanomedicine 2013;9:25-7. [PubMed]
- Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol 2005;50:N163-73.
- Robar JL. Generation and modelling of megavoltage photon beams for contrast-enhanced radiation therapy. Phys Med Biol 2006;51:5487-504. [PubMed]
- Robar JL, Riccio SA, Martin MA. Tumour dose enhancement using modified megavoltage photon beams and contrast media. Phys Med Biol 2002;47:2433-49. [PubMed]
- McMahon SJ, Mendenhall MH, Jain S, et al. Radiotherapy in the presence of contrast agents: a general figure of merit and its application to gold nanoparticles. Phys Med Biol 2008;53:5635-51. [PubMed]
- Garnica-Garza HM. Contrast-enhanced radiotherapy: feasibility and characteristics of the physical absorbed dose distribution for deep-seated tumors. Phys Med Biol 2009;54:5411-25. [PubMed]
- Kappus H, Bothe D, Mahmutoglu I. The role of reactive oxygen species in the antitumor activity of bleomycin. Free Radic Res Commun 1990;11:261-6. [PubMed]
- Mahmutoglu I, Scheulen ME, Kappus H. Oxygen radical formation and DNA damage due to enzymatic reduction of bleomycin-Fe(III). Arch Toxicol 1987;60:150-3. [PubMed]
- Wallach-Dayan SB, Izbicki G, Cohen PY, et al. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am J Physiol Lung Cell Mol Physiol 2006;290:L790-6. [PubMed]
- Ott M, Gogvadze V, Orrenius S, et al. Mitochondria, oxidative stress and cell death. Apoptosis 2007;12:913-22. [PubMed]
- Li JJ, Hartono D, Ong CN, et al. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010;31:5996-6003. [PubMed]
- Pan Y, Leifert A, Ruau D, et al. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009;5:2067-76. [PubMed]
- Chompoosor A, Saha K, Ghosh PS, et al. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small 2010;6:2246-9. [PubMed]
- Gao W, Xu K, Ji L, et al. Effect of gold nanoparticles on glutathione depletion-induced hydrogen peroxide generation and apoptosis in HL7702 cells. Toxicol Lett 2011;205:86-95. [PubMed]
- Zhang XD, Wu D, Shen X, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012;33:6408-19. [PubMed]
- Wu YN, Yang LX, Shi XY, et al. The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials 2011;32:4565-73. [PubMed]
- Tournebize J, Boudier A, Joubert O, et al. Impact of gold nanoparticle coating on redox homeostasis. Int J Pharm 2012;438:107-16. [PubMed]
- Menchón C, Martín R, Apostolova N, et al. Gold nanoparticles supported on nanoparticulate ceria as a powerful agent against intracellular oxidative stress. Small 2012;8:1895-903. [PubMed]
- Carter JD, Cheng NN, Qu Y, et al. Nanoscale energy deposition by X-ray absorbing nanostructures. J Phys Chem B 2007;111:11622-5. [PubMed]
- Misawa M, Takahashi J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV Irradiations. Nanomedicine 2011;7:604-14. [PubMed]
- Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res 2010;16:6139-49. [PubMed]
- Joh DY, Sun L, Stangl M, Al Zaki A, et al. Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One 2013; 30;8:e62425.


