
Efficacy of combination treatment modalities for intermediate and advanced hepatocellular carcinoma: intra-arterial therapies, sorafenib and novel small molecules
Introduction
Hepatocellular carcinoma (HCC) is a rising source of global morbidity and mortality. According to the World Health Organization, it is now second in producing cancer deaths in men (1). In nations showing the highest HCC prevalence, diagnosis of patients occurs at younger ages, though treatment may not be available (2). With no intervention, survival after diagnosis of intermediate to advanced HCC is approximately eight months (3); with expert care, prognosis can be extended beyond four years (4).
Treatment decisions are complex and dependent upon tumor staging, presence of portal hypertension, and the underlying degree of liver dysfunction, as well as local expertise. When HCC is confined to the liver with preserved hepatic reserve and no or minimal portal hypertension, a partial hepatectomy can be curative; however, recurrence, or de novo (metachronous) HCC is common. In patients with unresectable disease and tumor staging that falls within criteria, liver transplantation can be curative in a great majority of patients. Unfortunately, most patients will not be candidates for either surgery or transplant; clinicians also struggle with already cirrhotic patients with unresectable HCC who are not candidates for transplant. The use of combination therapy with surgical resection, as a pre-operative bridge to transplant, and with inpatients found to have lymphovascular invasion after transplant is an area of growing interest.
Locoregional treatments such as transarterial chemoembolization (TACE) or transarterial bead embolization (TABE) are generally used for intermediate disease, or Barcelona Clinic Liver Cancer B (BCLC B). Embolization of the vessels that supply HCC leads to a dense inflammatory response and necrosis of the lesion, although it often leaves a viable tumor along the periphery with documented vascular endothelial growth factor (VEGF) rebound (5). With these therapies, a partial response is common, as well as a high recurrence rate; combination with other modalities does not consistently yield survival rates greater than monotherapy (6).
The sequences that lead to the development of HCC are still incompletely understood, although the process likely begins with somatic mutations responsible for small tumor formation. The malignant hepatocytes release angiogenic growth factors (GFs) and tumor vascularization occurs allowing for expansion. In the pivotal phase III study, sorafenib, a small molecule multikinase inhibitor, was shown to extend overall survival by almost three months (7). Thus, current guidelines suggest its use in patients with advanced HCC (BCLC C) (8). Despite this critical step forward, poor outcomes continue to be the norm. The dominant molecular mechanistic aspect of sorafenib remains unclear. Which patients may benefit most from monotherapy is also not yet known. Although sorafenib was initially developed as a b-raf inhibitor for melanoma, it demonstrated little activity (9). It is likely that it inhibits c-raf that in turn decreases VEGF expression and cellular proliferation via MAPK, and induces apoptosis. VEGF is a central mediator of angiogenesis (10). It also appears to activate phosphatases, inhibit stat-3, and alter IL-6 signaling (11). Although sorafenib yields improvement in survival, adverse events are common which limit its use. The acceptable threshold of side effects may vary by clinician and patient; those providers with a greater comfort in dealing with common adverse effects such as hand-foot syndrome may ultimately have improved outcomes. Studies of sorafenib show that dose duration and amount of drug exposure are key to response (7).
Currently, most clinical trials for intermediate stage HCC pair an already established modality such as TACE or sorafenib with a novel drug. Although there are signs that these may offer small improvements over standard care, the results of this strategy are generally equivocal to date. Drug discovery and clinical trials should aim at tactical combinations of new agents that can continue in tandem with procedures like TACE. Drug resistance may be avoided through use of two or more small molecules sharing the same target, such as the molecules that inhibit the tyrosine kinase receptors. Horizontal or vertical targeting to signal pathways may also lead to synergistic anti-tumor effects. Unfortunately, there remain significant hurdles to overcome when attempting to combine drug therapies in early clinical development. Despite these challenges, combination therapy offers the opportunity for significant progress to be made. In this review, the rationale and obstacles for combination therapy in unresectable HCC will be discussed.
Obstacles in developing effective therapies: “heterogeneity of HCC”
Rationale for combined therapy: does one plus one equal two?
The Institute of Medicine recently summarized the rationale and need for combination treatments to accelerate cancer therapy development (12). The hope is that an appropriate combination of agents may be found that will allow for the best treatment effects with the least side effects (13). Single agent therapies may induce drug resistance, or only partially inhibit the molecular pathways involved. Combination therapy may produce more effective outcomes by targeting multiple pathways critical for cancer progression. This approach has proved highly effective in producing results against infectious diseases such as HIV and, more recently, HCV.
The development of novel combination therapies in HCC presents unique challenges. The most conspicuously obvious challenge is the diversity of conditions that lead to malignant transformation including chronic infection with hepatitis viruses, nonalcoholic steatohepatitis (NASH), hereditary diseases (hemochromatosis), toxins (alcohol and aflatoxin), and immune-mediated diseases (primary biliary cirrhosis and autoimmune hepatitis). Even within the various causes of HCC, the aberrance in the molecular pathways can be different (14). Patients may even have heterogeneity within a single tumor as well as synchronous and metachronous lesions. Genomic profiling of HCC highlights the diverse changes that can occur in HCC, although several discrete patterns can be recognized (15). Functional biological studies and biomarkers can guide clinical care to inform the selection of agents to improve these outcomes; however, many of these are lacking or are still in the early stages of development.
There is also concern from the FDA that novel-novel drug combinations may pose a greater risk to patients, although they may be supportive provided there is sufficient pre-clinical data. Additionally, combination therapy clinical trial design can prove complex; success may require significant pre-clinical data and planning (16). Drugs in combination have the potential to interact synergistically; this effect is lost when they are administered independently. In some cases, a single drug may have no direct effect on a disease, but when used in combination it may affect the metabolism of a second agent in a way that increases the overall effect. Careful consideration of the pharmacokinetics are required for successful phase I testing.
Potential drug trials combining novel agents are often complicated by economic and intellectual property considerations. Perception of considerable additional cost and risk to those in the private sector funding drug development also complicates matters. Furthermore, legal issues surrounding possible inventions derived from the collaboration can be a major sticking point from both academic and private institutions and may require lengthy negotiations. The development of trastuzumab emtansine (in the US, ado-trastuzumab emtansine), consisting of Genentech’s anti-HER2 monoclonal antibody trastuzumab conjugated to Immunogen’s anti-mitotic agent mertansine, which is now approved for metastatic breast cancer, is a model to overcome these obstacles through use of a collaborative and successful approach (17). The irreversible binding of trastuzumab to the HER-2 receptor leads to internalization of mertansine by the tumor cell. The use of this chimeric small molecule showed improved efficacy in patients who had already received trastuzumab (18). Strategies such as these in HCC may decrease the toxicity and increase the efficacy of novel therapeutics.
Targeted therapy today: duck hunting with a bow and arrow
Complex cellular biology with a set of heterogeneous causes is responsible for challenging drug development in HCC. The hallmark of HCC is its dense hypervascular arterial blood supply; consequently, angiogenesis pathways are of pronounced interest. HCC’s can be linked to genetic mutations and epigenetic alterations in the cell cycle, proliferation of cells, and production of GFs. Although the exact sequence of hepatocarcinogenesis is not known, good evidence exists that at least three distinct molecular pathways are dysregulated: the mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase (PI3K), and β-catenin (See Figure 1). Thus, therapy targeting a single aspect of HCC’s molecular biology will likely be met with limited success. A cocktail of small molecules targeting the overlapping and alternate pathways may help overcome the limitations encountered thus far.
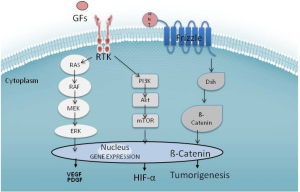
Sorafenib appears to have multiple effects in vitro. Most prominently, it inhibits the Raf family kinases through the MAPK pathway activated by VEGF (19). This is believed to alter cellular proliferation, reduce angiogenesis, and increase apoptosis in tumor cells (20). Sorafenib decreases mRNA expression of VEGF via inhibition of the PI3K pathways in tumor cells, and inhibits the VEGF receptor kinase in the endothelial cell (21). Wnt signaling is identified as a key player in many solid tumors, including HCC. Activating mutation in the β-catenin gene (CTNNB1) represents the second highest frequency of known mutations in HCC (22). In HepG2 cells, which harbor this mutation, sorafenib attenuates Wnt-pathway activation (23). Historically, developments of agents that target Wnt directly are complicated by toxicity; however, there are some Wnt antagonists such as LGK974 in early development (24). Levels of C-Kit and HGF may predict higher or lower responses to sorafenib (25).
Although oncogenic mutations are responsible for the initiation of tumor growth, GFs are the major regulators of all subsequent steps of tumor progression. Tumors that produce excessive GF can manipulate their own further growth through autocrine regulation, as well as support metastatic growth in a paracrine manner. Platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) also lead to activation of the Ras-Raf-MAP-ERK pathway and expansion mediators of mutation-bearing clones. Sunitinib inhibits both VEGF and PDGF signaling cascades (26), although early clinical trials were stopped as survival in patients treated with sorafenib alone was superior (27). Linifanib is another VEGFR/PDGFR multikinase inhibitor in clinical trials for HCC. Some data suggest that resistance to tyrosine kinase inhibitors is mediated through FGFR; thus the use of brivanib, which targets FGF in addition to the VEGF cascade, has a theoretical role in sorafenib-resistant HCC (28). The monoclonal antibody bevacizumab offers a different approach: irreversibly binding systemic VEGF in a protein complex, thus blocking it from binding to the receptor (29). Ramucirumab works by binding to VEGF receptor 2 (VEGFR2), thus blocking the binding of VEGF to VEGFR2 (30).
Hepatocyte growth factor (HGF) is a ligand that appears crucial for HCC progression (31). It binds to the c-Met receptor, another tyrosine kinase receptor linked to the effector pathways of HCC. C-met leads to downstream activation of MAPK and PI3K. The PI3K/Akt/mTOR pathway leads to activation of a number of nuclear transcription factors, including nuclear factor kappa from B cells (NF-κB). The mTor inhibitors everolimus, sirolimus and temsirolimus are drugs that have the potential to alter this pathway. There are several drugs in development that target c-Met, including tivantinib, cabozantinib, and foretinib. As evidenced by tumor immunohistochemistry, use of tivantinib in high c-Met expresser patients appears to improve the drug’s effectiveness (32), although serious adverse events, including death, were reported in the initial trials (33). Future studies of tivantinib should be accompanied by tumor sampling to determine c-Met expression since it appears to predict response.
HCC drug combination therapy could involve two small molecules with the same target, different targets on the same pathway (vertical), or inhibition of different pathways (horizontal). For example, dual treatment with tyrosine multikinase inhibitors like sorafenib and brivanib could potentially further reduce VEGF signaling and resistance. Using oral therapies with radiation therapy, surgery, transarterial therapy, or thermal ablation are all potential combinations for our patients in the clinic setting.
HCC model systems
Model systems for HCC allow researchers to understand the effects of small molecules in vitro, and identify which may perform synergistically. A number of different model systems exist to study HCC in the laboratory; each has certain limitations. Immortalized human hepatocytes can be obtained by in vitro transfection of SV-40 T antigen (34), and by overexpression of the HCV core protein (35). Most studies are currently performed with cells extracted from primary tumors suitable for maintaining characteristics after multiple passages such as HepG, Hep3B and Huh7. These cell lines are derived from human tumors and, thus, reflect the genomic defects and pathophysiology of a single individual. For example, Huh7 cells were established in 1982 from a 57-year-old Japanese male (36). Despite the inherent difficulty in interpreting the results of these studies, they can provide useful preclinical data for evaluating the synergy of combination therapy. Furthermore, many studies have shown favorable results with combinations of several anti-HCC small molecules, validating the performance of dual inhibition of HCC-related biomarkers such as VEGF or Akt phosphorylation (37). Subcutaneous xenograft models using implanted tumor tissue in mice are used to characterize the performance of various molecules in HCC. Although they are labor intensive, expensive, and technically challenging, they can be useful in predicting therapies that may perform synergistically in clinical trials.
Hepatitis viruses
The most common causes of HCC are the hepatitis B virus (HBV) and the hepatitis C virus (HCV) which account for 80% of all cases (38). HBV is a DNA virus that can integrate into host DNA and has the oncogenic potential to transform hepatocytes in the absence of cirrhosis or even significant fibrosis (39); the HBx protein also shows oncogenic potential. HCV is almost exclusively linked to HCC in cirrhosis and may exert its oncogenesis through viral proteins such as the core (40). Among patients with NASH, HBV and HCV, risk of HCC varies by viral genotype, presence of core and precore mutations and other risk factors such as gender and concurrent alcohol use. In the key study by Liaw, suppression of HBV with antivirals was shown to decrease the risk of HCC in patients with cirrhosis (41). In this study, patients with HBV cirrhosis were treated with lamivudine or placebo. The data and safety monitoring board (DSMB) terminated the study at 32 months because the lamivudine group fared better, showing a modest reduction of HCC. Recent studies with tenofovir and entecavir have shown similar results (42).
In HCV, only durable cures from infection are linked to a reduction in HCC risk (43). Much enthusiasm was generated in recent years as HCV direct-acting antivirals arrived in the market. At the time of publication of this manuscript, interferon remains the backbone of treatment. Due to adverse events and continued poor response to therapy, most clinicians avoid treating patients with HCC. In the HALT-C trial, there was no reduced risk of HCC after prolonged IFN therapy (44). Thus, a partial response is not protective. There is a universal consensus that patients who achieve an SVR have a reduced risk of HCC (45). Antiviral therapy may turn off inflammation and decrease the risk of HCC. Whether treatment with antivirals can decrease progression of already present HCC remains unanswered. Since HCV’s viral proteins are known to modulate important pathways related to HCC, removal of these instigators may slow progression in the same way as other inhibitors. Currently, sofosbuvir is being tested in patients with HCC awaiting liver transplant; however, progression of HCC is not a viable endpoint as patients will be transplanted throughout the study. Clinical trials examining anti-HCV treatment in patients with HCC who are not transplant candidates will be valuable.
HCC therapies in development
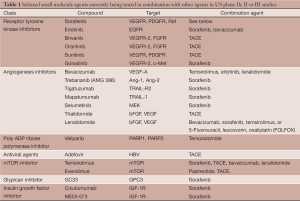
Most small molecules currently in phase Ib, II and III trials in combination have failed to show non-inferiority or superiority to sorafenib monotherapy. The vast majority of the upcoming or ongoing trials with these agents seek to pair them with sorafenib or TACE (See Table 1); however, there may also be a role for small molecules as adjuvant therapy with resection or around time of liver transplant.
Full table
Adjuvant therapy for resection and liver transplant
No studies have been performed using small molecules as neoadjuvant therapy before resection. Resection is generally considered curative and thus the use of drugs like sorafenib would be aimed at decreasing the chance of recurrence. In this setting, treatment of viral hepatitis would appear to be the most important therapy to decrease risk of HCC recurrence. Interferon therapy has been shown to decrease recurrence and mortality in patients with HBV/HCV-related HCC in meta-analyses (46,47). Whether sorafenib has benefit in patients after curative resection is the topic of the phase III trial STORM (Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma) which has completed recruitment, with results expected soon. Many in clinical practice may use sorafenib in patients who were found to be beyond Milan criteria on the basis of the explanted liver, but no prospective data exists to support this practice. Two small retrospective studies showed increased overall survival in patients treated with sorafenib when transplanted beyond Milan criteria, and suggest a benefit of such practice (48,49).
Sorafenib and TACE: one + one = one
Historically, there was insufficient data for an evidenced-based guideline on the combined use of TACE and sorafenib. Thus, clinicians who incorporated this into their practice did so empirically. The theoretical combination approach of embolization techniques with sorafenib was aimed at the possibility that sorafenib could slow the revascularization that occurs after embolization. Once these two modalities were placed in the hands of clinicians, adverse events were common. Additionally, some experts felt that pretreatment with sorafenib made embolization more difficult, and that larger chemo particles (>400 microns) were not adequately permitted to enter the tumor due to anti-VEGF therapy. These reports were attributed to the continuous anti-angiogenesis therapy with sorafenib. Additionally, use of TACE and sorafenib concurrently could possibly worsen adverse events related to variceal bleeding, hand foot syndrome (HFS), and hypertension. Due to the theoretical reasons mentioned, approaches were developed to temporally separate the two treatments.
As a result, sequential and interrupted strategies were advanced clinically and used in trials (50). Sequential therapy involves only starting sorafenib after TACE sessions are complete. The interrupted strategy starts sorafenib after the TACE session, and stops when more TACE is planned. The Space Study had sorafenib started before TACE, but therapy was halted for one week before and at least three days afterward, thus decreasing the possibility that sorafenib would interrupt VEGF signaling or cause other signaling changes. The main benefit of the interrupted approach as opposed to the continuous approach is the possible decreased risk of variceal bleeding. Unfortunately, no randomized data is available using the interrupted strategy, and conflicting data exists on the safety of continuous treatment (51). Sequential therapy may offer strategic opportunities to alter angiogenesis, although there is no clear evidence to recommend this as a strategy. Additionally, the agents and concentrations of chemotherapy used vary by center, as does the use of doxorubicin bead-TACE (DEB-TACE). Most experts agree that the key intervention performed is the embolization of the vessel, with less importance assigned to the chemotherapy used. Three high-quality studies (randomized, double-blind, and placebo-controlled) were performed, and the comparative analysis of these studies shows the heterogeneity of patients, study designs, variability of endpoints, and TACE protocols in recent HCC trials. Additionally, the dosing reductions and sequence of sorafenib varied from study to study.
In a Japanese and Korean phase III study of patients with unresectable HCC, Kudo et al. reported on 458 patients with intermediate HCC randomized to receive 400 mg b.i.d. of sorafenib or placebo after TACE (52). The primary endpoint was time to progression (TTP). Most patients started on sorafenib/placebo nine weeks after TACE using a sequential strategy; the median dose of sorafenib administered was 386 mg per day. High rates of dose reduction (73%) and interruption (91%) were seen in this study. Median TTP in the sorafenib and placebo groups was 5.4 and 3.7 months, respectively [hazard ratio (HR), 0.87; 95% confidence interval (CI), 0.70-1.09; P=0.252]. Despite the lack of efficacy, some suggest that this was related to significant reductions in sorafenib and high rates of adverse events compared with other studies. In the subgroup analysis of this study, Korean patients underwent longer sorafenib treatment duration and achieved significantly prolonged TTP (HR 0.38; 95% CI, 0.18-0.81).
Although the SHARP trial was not designed to assess the performance in sorafenib specific to certain liver diseases (7), post-hoc sub-group analysis showed HCV patients treated with sorafenib had significantly longer overall survival compared to those treated with placebo (14 vs. 7.9 months; HR 0.50; 95% CI, 0.32-0.77) (53). In a single-center study conducted in 2007 prior to knowledge of the SHARP results, 62 HCV-positive patients with BCLC B HCC sequentially received either sorafenib (400 mg b.i.d.) or placebo 30 days after TACE (54). The primary endpoints were TTP and safety. The median TTP was 9.2 months in the sorafenib group and 4.9 months in the placebo group (HR 2.5; 95% CI, 1.66-7.56).
In the Sorafenib or Placebo in combination with TACE (SPACE) trial, 307 patients with intermediate-stage HCC received sorafenib, 400 mg b.i.d., or placebo continuously in combination with DEB-TACE. The primary endpoint was TTP in this phase II study. A trend toward prolonged TTP emerged with the sorafenib group compared to the placebo group (HR 0.79; 95% CI, 0.58-1.08); although median TTP was slightly better in the placebo group (5.6 vs. 5.5 months) (55). The dose interruption of sorafenib may explain the lack of response.
Although there continues to be significant interest in the combination of TACE and sorafenib, probably because of the general familiarity with each modality, efficacy has not been clearly shown, and data are currently inconclusive. The side effects with combined use of TACE and sorafenib are acceptable. Combined toxicity profiles are similar to those seen with either drug alone although there may be a potential additive toxicity in regards to HFS and hypertension. Further data is needed in regards to BCLC B and C. Randomized controlled trials are lacking; currently the combination of TACE and sorafenib does not appear additive.
Combination of sorafenib and chemotherapeutic agents
Theoretically, the combined use of sorafenib with chemotherapeutic agents that inhibit the MAPK pathway may reduce resistance to sorafenib (56). In a randomized trial comparing doxorubicin alone vs. in combination with sorafenib, patients receiving sorafenib fared significantly better in regards to TTP (6.4 vs. 2.8 months, P=0.02) (57). As there was no arm with sorafenib monotherapy, the authors could not conclude that the combination of doxorubicin and sorafenib proved better than sorafenib alone. The combination of sorafenib, capecitabine, and oxaliplatin compared to sorafenib monotherapy is currently being tested in a phase III trial based on early data showing favorable response rates (58).
Potential use of transarterial embolization
Transarterial radioembolization (TARE) with Yttrium-90 loaded microspheres is a form of brachytherapy. The procedure appears to be safe and efficacious in patients with unresectable HCC, although it does not appear superior to TACE based on preliminary data from non-randomized studies (59). TACE is associated with increased risk of ischemic-related necrosis in patients with portal vein thrombosis (PVT) (60) whereas TARE causes only minimal alteration in vascularity (microembolization) (61). In retrospective and non-randomized prospective studies TACE and TARE have generally shown similar performance although there seems to be reduced toxicity in TARE patients (59,62-65). Again, these trials suffer from inferior design and lack of uniformity in the TACE and TARE procedures making comparisons between studies difficult. Studies of TABE combination therapy with sorafenib are in progress as well. To date, no data exists on the combination of TARE and systemic therapy. Intriguingly, in vitro studies suggest that treatment with sorafenib re-sensitized radiation induces resistance via effects on Raf-1 (66). Given TARE’s favorable side effect profile and potential mechanistic advantage of dual use, a well-designed trial testing the combination of TARE and sorafenib (or other systemic agents) would be worthwhile (67).
Failure of other tyrosine kinase inhibitors therapies: sunitinib, brivanib, and linifanib
Two other tyrosine kinase inhibitors, sunitinib and brivanib, were compared to sorafenib; neither demonstrated superiority. In the case of sunitinib, the trial stopped early due to statistically significant reduced survival in the sunitinib arm (27). Brivinib is another multikinase inhibitor that inhibits VEGF and FGF. In the phase III BRISK-FL trial, sorafenib and brivanib monotherapy were compared. Overall survival was similar so the trial did not meet its primary endpoint of non-inferiority to sorafenib. The side effect profile of sorafenib was slightly more favorable, making brivanib even less desirable as monotherapy (68). The disappointing results of these promising agents demonstrate the need for novel trial design. Whether these drugs might have improved outcomes if used sequentially or in combination with sorafenib or each other remains unknown. Linifanib is another potent inhibitor of the tyrosine kinase receptor that failed to show non-inferiority in comparison to sorafenib (69). As discussed earlier, dual tyrosine kinase inhibitor use has the potential to reduce resistance although to date these combinations have not been explored. Phase III trials to evaluate the combination of TACE and sunitinib or TACE and brivanib have been proposed; however, there is no rationale to believe these combinations would perform better in this regard than sorafenib and these studies may ultimately not be completed.
Diversifying targets in HCC to attempt success
A number of other small molecules that target other aspects of HCC molecular pathogenesis are in development. A small phase II trial of erlotinib, an epithelial growth factor receptor (EGFR) inhibitor, showed an impressive 13-month survival in a patient with advanced HCC (70). The SEARCH (Sorafenib and Erlotinib, a rAndomized tRial protoCol for the treatment of patients with HCC) showed no advantage of combined treatment with sorafenib. Studies of erlotinib and bevacizumab combined therapy did not show improved outcomes to the historical controls from SEARCH (71). Bevacizumab is a humanized monoclonal antibody that inhibits vascular endothelial growth factor (VEGF) which is a key driver of angiogenesis. In a phase II clinical trial it showed a favorable response but there are no further studies evaluating efficacy (72). In a recent single center study of TACE and bevacizumab compared to TACE alone, patients who received bevacizumab showed increased progression-free survival (P=0.021), although there was no difference in overall survival between the two groups (73).
The mTor pathway has been identified as a target in a number of malignancies. In early phase I/II studies, everolimus monotherapy shows antitumor effects along with a reasonable safety profile (74). Combination studies of everolimus and sorafenib are ongoing. Rapamycin and temsirolimus also have the potential to be used against HCC. Other novel molecules such as trebananib are currently in early development. Trebananib targets angiopoietin signaling at later stages of vessel maturation and demonstrates synergy when combined with VEGF inhibitors in pre-clinical studies (75). This molecule will be tested in combination with sorafenib in an upcoming phase II trial.
Trial design for combination therapies
Review of the available literature demonstrates the paucity of combination therapy clinical trials undertaken outside of those attempting to add sorafenib or TACE to an agent. The principal benefits of combination therapy are to exploit synergy and differential susceptibility of tumors to agents, and to utilize non-overlapping toxicities (76). Although there would appear to be much benefit from this approach, investigators cannot automatically assume that two safe drugs will remain as such when used in combination. Traditionally, in phase I testing of two agents in combination, the maximum tolerated dose (MTD) must be determined without regard to efficacy. Most experts now consider that an adaptive approach to combination therapy is beneficial due to the multiple variables involved in trial design. The main aim of these trials is to establish safety while dose escalating and maximizing the anti-tumor response (77). By their nature, these types of trials are much more complex to analyze statistically; as such, careful design consideration is needed.
Conclusions and future directions
Combination therapy for HCC is a promising avenue for patients with advanced HCC. There are many potential modalities and agents to explore as possible therapies; however, these need to be approached uniformly to enable appropriate data interpretation. As a community, we must move beyond sorafenib and TACE. Upcoming clinical trials should focus on inhibition of multiple targets based on preclinical data from basic scientists. The approach taken with HIV and HCV (in which multiple pathways for inhibition of viral replication are undertaken) could prove beneficial if applied to cancer pathogenesis. Furthermore, academic and industry leaders must establish new partnerships to facilitate testing of these new therapies in tandem. More research on individual therapy for patients is necessary. Fine needle aspiration of HCC and the tumor microenvironment along with genomic profiling may lead to more targeted therapy, thereby improving outcomes in clinical trials. Sampling of tumors may also allow for stratifying patients by biomarkers like c-Met.
Acknowledgments
The authors would like to express their gratitude to Dr. Lark Lands for her invaluable assistance in preparing the manuscript for publication.
Funding: JAG was supported by NIH grant 5T32DK007202-39.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Catherine T. Frenette) for the series “Liver Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.10.01). The series “Liver Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Kew MC. Hepatocellular carcinoma in African Blacks: Recent progress in etiology and pathogenesis. World J Hepatol 2010;2:65-73. [PubMed]
- . A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [PubMed]
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 2012;56:1330-5. [PubMed]
- Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci 2008;99:2037-44. [PubMed]
- Dufour JF, Bargellini I, De Maria N, et al. Intermediate hepatocellular carcinoma: current treatments and future perspectives. Ann Oncol 2013;24:ii24-9. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [PubMed]
- Mangana J, Levesque MP, Karpova MB. Sorafenib in melanoma. Expert Opin Investig Drugs 2012;21:557-68. [PubMed]
- Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306-9. [PubMed]
- Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006;5:835-44. [PubMed]
- LoRusso PM, Canetta R, Wagner JA, et al. Accelerating cancer therapy development: the importance of combination strategies and collaboration. Summary of an Institute of Medicine workshop. Clin Cancer Res 2012;18:6101-9. [PubMed]
- Mcarthur GA, Ribas A. Targeting oncogenic drivers and the immune system in melanoma. J Clin Oncol 2013;31:499-506. [PubMed]
- Hoshida Y, Moeini A, Alsinet C, et al. Gene signatures in the management of hepatocellular carcinoma. Semin Oncol 2012;39:473-85. [PubMed]
- Nishida N, Goel A. Genetic and epigenetic signatures in human hepatocellular carcinoma: a systematic review. Curr Genomics 2011;12:130-7. [PubMed]
- Humphrey RW, Brockway-Lunardi LM, Bonk DT, et al. Opportunities and challenges in the development of experimental drug combinations for cancer. J Natl Cancer Inst 2011;103:1222-6. [PubMed]
- Ballantyne A, Dhillon S. Trastuzumab emtansine: first global approval. Drugs 2013;73:755-65. [PubMed]
- Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2013;31:1157-63. [PubMed]
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006;66:11851-8. [PubMed]
- Newell P, Toffanin S, Villanueva A, et al. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol 2009;51:725-33. [PubMed]
- Gedaly R, Angulo P, Hundley J, et al. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res 2010;30:4951-8. [PubMed]
- Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res 2008;68:6779-88. [PubMed]
- Lachenmayer A, Alsinet C, Savic R, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res 2012;18:4997-5007. [PubMed]
- Lum L, Clevers H. Cell biology. The unusual case of Porcupine. Science 2012;337:922-3. [PubMed]
- Llovet JM, Peña CE, Lathia CD, et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2012;18:2290-300. [PubMed]
- Allen E, Walters IB, Hanahan D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin Cancer Res 2011;17:5299-310. [PubMed]
- Cheng A, Kang Y, Lin D, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2011;29:abstr 4000.
- Villanueva A, Llovet JM. Second-line therapies in hepatocellular carcinoma: emergence of resistance to sorafenib. Clin Cancer Res 2012;18:1824-6. [PubMed]
- . Bevacizumab. Anti-VEGF monoclonal antibody, avastin, rhumab-VEGF. Drugs R D 2002;3:28-30. [PubMed]
- Krupitskaya Y, Wakelee HA. Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr Opin Investig Drugs 2009;10:597-605. [PubMed]
- Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res 2013;19:2310-8. [PubMed]
- Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol 2013;14:55-63. [PubMed]
- Santoro A, Simonelli M, Rodriguez-Lope C, et al. A phase-1b study of tivantinib (ARQ 197) in adult patients with hepatocellular carcinoma and cirrhosis. Br J Cancer 2013;108:21-4. [PubMed]
- Fukaya K, Asahi S, Nagamori S, et al. Establishment of a human hepatocyte line (OUMS-29) having CYP 1A1 and 1A2 activities from fetal liver tissue by transfection of SV40 LT. In Vitro Cell Dev Biol Anim 2001;37:266-9. [PubMed]
- Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology 2000;271:197-204. [PubMed]
- Nakabayashi H, Taketa K, Miyano K, et al. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res 1982;42:3858-63. [PubMed]
- Chan SL, Wong CH, Lau CP, et al. Preclinical evaluation of combined TKI-258 and RAD001 in hepatocellular carcinoma. Cancer Chemother Pharmacol 2013;71:1417-25. [PubMed]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-1273.e1.
- Tsai WL, Chung RT. Viral hepatocarcinogenesis. Oncogene 2010;29:2309-24. [PubMed]
- Fishman SL, Factor SH, Balestrieri C, et al. Mutations in the hepatitis C virus core gene are associated with advanced liver disease and hepatocellular carcinoma. Clin Cancer Res 2009;15:3205-13. [PubMed]
- Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521-31. [PubMed]
- Singal AK, Salameh H, Kuo YF, et al. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther 2013;38:98-106. [PubMed]
- Singal AK, Singh A, Jaganmohan S, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol 2010;8:192-9. [PubMed]
- Lok AS, Everhart JE, Wright EC, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology 2011;140:840-9; quiz e12.
- van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584-93. [PubMed]
- Wang J, He XD, Yao N, et al. A meta-analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Can J Gastroenterol 2013;27:351-63. [PubMed]
- Zhuang LP, Zeng XT, Meng ZQ. A systematic review and meta-analysis of randomized controlled trails: adjuvant interferon therapy for hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2012;20:363-7. [PubMed]
- Teng CL, Hwang WL, Chen YJ, et al. Sorafenib for hepatocellular carcinoma patients beyond Milan criteria after orthotopic liver transplantation: a case control study. World J Surg Oncol 2012;10:41. [PubMed]
- Saab S, Mctigue M, Finn RS, et al. Sorafenib as adjuvant therapy for high-risk hepatocellular carcinoma in liver transplant recipients: feasibility and efficacy. Exp Clin Transplant 2010;8:307-13. [PubMed]
- Strebel BM, Dufour JF. Combined approach to hepatocellular carcinoma: a new treatment concept for nonresectable disease. Expert Rev Anticancer Ther 2008;8:1743-9. [PubMed]
- Sieghart W, Pinter M, Reisegger M, et al. Conventional transarterial chemoembolisation in combination with sorafenib for patients with hepatocellular carcinoma: a pilot study. Eur Radiol 2012;22:1214-23. [PubMed]
- Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-27. [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [PubMed]
- Sansonno D, Lauletta G, Russi S, et al. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist 2012;17:359-66. [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. J Clin Oncol 2012:30:abstr LBA154^.
- Mccubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 2006;46:249-79. [PubMed]
- Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA 2010;304:2154-60. [PubMed]
- Chong DQ, Tan IB, Choo SP, et al. The evolving landscape of therapeutic drug development for hepatocellular carcinoma. Contemp Clin Trials 2013;36:605-15. [PubMed]
- Moreno-Luna LE, Yang JD, Sanchez W, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol 2013;36:714-23. [PubMed]
- Jelic S, Sotiropoulos GCESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v59-64. [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [PubMed]
- Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8. [PubMed]
- Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010;21:224-30. [PubMed]
- Carr BI, Kondragunta V, Buch SC, et al. Therapeutic equivalence in survival for hepatic arterial chemoembolization and Yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 2010;116:1305-14. [PubMed]
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-507.e2.
- Tang WY, Chau SP, Tsang WP, et al. The role of Raf-1 in radiation resistance of human hepatocellular carcinoma Hep G2 cells. Oncol Rep 2004;12:1349-54. [PubMed]
- Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464-73. [PubMed]
- Johnson P, Qin S, W PJ, et al. Brivanib (BRI) versus sorafenib (SOR) as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma (HCC): results from the phase 3 BRISK-FL study [abstract LB-6]; Proceedings of the 63rd Annual Meeting of the American Association for the Study of Liver Diseases; November 9-13, 2012; Boston, MA, USA.
- Calin Cainap, Qin S, Huang WT, et al. Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2012;30:abstr 249.
- Philip PA, Mahoney MR, Allmer C, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular Cancer. J Clin Oncol 2005;23:6657-63. [PubMed]
- Govindarajan R, Siegel E, Makhoul I, et al. Bevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinoma. Am J Clin Oncol 2013;36:254-7. [PubMed]
- Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 2008;26:2992-8. [PubMed]
- Britten CD, Gomes AS, Wainberg ZA, et al. Transarterial chemoembolization plus or minus intravenous bevacizumab in the treatment of hepatocellular cancer: a pilot study. BMC Cancer 2012;12:16. [PubMed]
- Zhu AX, Abrams TA, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer 2011;117:5094-102. [PubMed]
- Gerald D, Chintharlapalli S, Augustin HG, et al. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res 2013;73:1649-57. [PubMed]
- Korn EL, Simon R. Using the tolerable-dose diagram in the design of phase I combination chemotherapy trials. J Clin Oncol 1993;11:794-801. [PubMed]
- Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major Cancer signaling pathways. J Clin Oncol 2013;31:1592-605. [PubMed]


