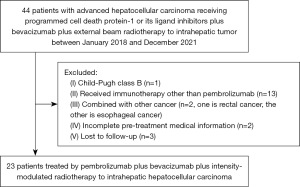
Liver-directed moderately hypo-fractionated radiotherapy combined with pembrolizumab and bevacizumab for advanced hepatocellular carcinoma: a retrospective observational study of 23 cases
Highlight box
Key findings
• Integrating radiotherapy into bevacizumab-pembrolizumab was preliminarily an effective regimen for advanced hepatocellular carcinoma (HCC). This tri-modal therapy brought about more treatment related adverse events (TAREs) which were expected and manageable.
What is known and what is new?
• Programmed cell death protein 1 (PD-1) or its ligand (PD-L1) monoclonal antibody combined with bevacizumab has been established as first-line systemic treatment for advanced HCC. Radiotherapy is a crucial local treatment for HCC. However, whether radiotherapy can enhance efficacy of anti-PD-1 immunotherapy plus bevacizumab for HCC remains unclear.
• Combining radiotherapy with bevacizumab and pembrolizumab was a viable and effective therapeutic choice for advanced HCC.
What is the implication, and what should change now?
• Patients with advanced HCC may benefit from the combination therapy of radiotherapy, bevacizumab and pembrolizumab. This tri-modal regimen may be a potential conversion therapy for unresectable, locally advanced HCC.
Introduction
Worldwide, liver cancer is the sixth most common emerging tumor, and the third leading cause of cancer-related death. Hepatocellular carcinoma (HCC) is the main pathological type of primary liver cancer, comprising 75–85% of cases. Hepatitis B virus or hepatitis C virus infection is the predominant etiology (1). Prognosis of HCC is very poor, with 5-year overall survival (OS) approximately equal to 18% (2). Up to 30–35% of cases are diagnosed as advanced stage, to whom systemic treatment is recommended (2-3).
Programmed cell death protein 1 (PD-1) or its ligand (PD-L1) inhibitors have shown clinical benefits in various cancers, including HCC. Only 12.4–18.3% of HCC patients benefit from PD-1/PD-L1 inhibitor monotherapy regardless of in first-line or second-line setting. However, objective response rate (ORR) increased to 20.5–37% when PD-1/PD-L1 inhibitor was combined with anti-angiogenesis therapy (including small molecular tyrosine kinase inhibitors and bevacizumab) (4). Sintilimab, a PD-1 monoclonal antibody, and atezolizumab, a PD-L1 monoclonal antibody, have been approved in combination with bevacizumab as standard first-line systemic treatment for advanced HCC (4-6) in China mainland.
Radiotherapy has become an important local treatment for HCC. There are increasing evidences supporting complex cross-talk between radiotherapy, tumor vasculo-genesis and anti-tumor immunity. Irradiation causes aging and apoptosis of vascular endothelial cells, collapse of blood vessels, and results in local hypoxia which stimulates the expression of angiogenesis molecules contributing to radio-resistance in turn (7). Bevacizumab can reduce hypoxia and alleviate resistance to irradiation through blocking angiogenesis signaling and normalizing tumor vessels (8,9). Anti-angiogenesis treatment promotes macrophage M1 polarization, CD8+ T cell infiltration and secretion of anti-tumor cytokine, including CXCL9, CXCL10, CCL21 and IFN-γ (10,11). Local irradiation to a tumor lesion can result in a non-irradiated tumor shrink by enhancing anti-cancer immune response, which is called abscopal effect (12). The mechanism lies in that irradiation induces immunogenic death of tumor cells, releasing damage-associated molecular patterns (DAMPs), such as calreticulin, adenosine triphosphate, and high mobility group box 1, and tumor-associated antigens (TAAs) which increase the activity of antigen-presenting cells (APCs) and specific T cells (12,13). Radiation-induced damage also increases availability of double-stranded DNA in the cytoplasm, and activates the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)-stimulator of interferon genes (STING) pathway which promotes production and secretion of cytokines and chemokines, recruiting and activating immune cells (13). Based on the mechanism mentioned above, it is rational to integrate radiotherapy, anti-PD-1/PD-L1 immunotherapy and bevacizumab. And this combination therapy has benefited patients with recurrent high-grade glioma in a prospective phase I clinical trial where tri-modal therapy of radiotherapy plus bevacizumab plus pembrolizumab achieved an ORR of 78%, even in patients with negative PD-L1 expression and/or resistance to bevacizumab. The median OS was 13.5 and 9.3 months for bevacizumab-naïve patients and bevacizumab-resistant patients, respectively (14). Zhong et al. retrospectively reported ORR of 40% and disease control rate (DCR) of 86.7% in a combination treatment consisting of palliative radiotherapy, anti-angiogenesis therapy and PD-1/PD-L1 inhibitors for advanced HCC with expected and manageable safety profiles (15). However, it is still uncertain whether adding intrahepatic tumor-directed radiotherapy to the combination of bevacizumab plus PD-1/PD-L1 inhibitor could further improve treatment outcome of advanced HCC. We conducted this retrospective observational study to provide data on efficacy and safety of liver-directed external beam moderately hypo-fractionated radiotherapy combined with bevacizumab (a monoclonal antibody targeting vascular endothelial growth factor) and pembrolizumab (a PD-1 monoclonal antibody) for advanced HCC. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1333/rc).
Methods
Patients’ eligibility
We retrospectively reviewed medical records of advanced HCC patients treated in the Fifth Affiliated Hospital of Sun Yat-sen University from January 2018 to December 2021. Patients with advanced HCC who met the following criteria were enrolled into this study: (I) age was 18–70 years old; (II) had received combined treatment including external beam hypo-fractionated radiotherapy to intra-hepatic tumor, bevacizumab and pembrolizumab; (III) radiation was started within 2 wteeks before or after the first administration of pembrolizumab; (IV) bevacizumab was initiated concurrently with pembrolizumab; (V) liver function was Child-Pugh A class. Patients who had received liver transplantation or had other malignancies were excluded. Anti-viral treatment was administered to patients with active hepatitis virus infection. This study was approved by Ethics Board of the Fifth Affiliated Hospital of Sun Yat-sen University (No. K47-1). The informed consent on treatment was taken from all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data collection and study objectives
Data on sex, age, hepatitis virus infection, Eastern Cooperative Oncology Group (ECOG) score, α-fetoprotein (AFP) level, Child-Pugh score, macrovascular invasion, extra-hepatic metastasis, number of tumor nodule, tumor size, previous treatment, pembrolizumab and its treatment cycles, bevacizumab and its treatment cycles, radiotherapy information, treatment-related adverse events (TRAEs), efficacy and survival were collected. TRAEs were graded according to the US National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE v4.03), with maximum grade recorded.
The primary endpoint was ORR. The secondary endpoints were progression-free survival (PFS), OS, DCR, duration of overall response (DOR) and toxicity profile. Treatment response evaluation was according to Response Evaluation Criteria in Solid Tumor (RECIST) v 1.1 (16). The definition of endpoints was as follow: (I) ORR, the proportion of patients with complete or partial response that was maintained for at least four weeks from the first radiological confirmation; (II) PFS, the time from the commencement of treatment to progression or death; (III) OS, the time from the commencement of treatment to death from any cause; (IV) DCR, the proportion of patients with objective response or stable disease; (V) DOR, the time from the first radiology-confirmed overall response to progression.
Treatment
Radiotherapy
Irradiation plan was calculated by intensity-modulated radiotherapy (IMRT) technique. Totally, 45 Gy was prescribed to tumor in a moderately hypo-fractionated scheme, with 3.0 Gy per fraction. The biological equivalent dose (BED) was 54–58.5 Gy (tumorous α/β =10–15 Gy). Patients were irradiated once a day from Monday to Friday.
Pembrolizumab and bevacizumab
Pembrolizumab was infused intravenously at a dose of 200 mg, every 3 weeks, according to KEYNOTE-240 trial (17). Bevacizumab was administered intravenously over 90 minutes by 15 mg/kg bodyweight every 3 weeks (5). Pembrolizumab and bevacizumab were infused within 2 weeks before or after the start of radiotherapy.
Statistical analysis
All statistical analysis were performed by SPSS Statistics, Version 25.0 (SPSS Inc., Chicago, IL, USA). ORR and DCR were calculated with 95% confidence interval (CI) by Clopper-Pearson method. Survival outcomes were estimated by Kaplan-Meier method. Double-tailed P value <0.05 was considered significant.
Results
Patients and treatment information
Twenty-three patients were eligibly enrolled into this study (Figure 1). The clinicopathological characteristics and treatment information are summarized in Table 1. Median age was 48 years (ranges, 24–67 years). The enrolled population was predominantly male (22/23, 95.7%). Twenty-two patients had Barcelona Clinic Liver Cancer (BCLC) staging C disease, with median and mean tumor diameters equal to 9.7 (range, 3.1–15.0) cm and 8.6 (95% CI, 5.2–12.1) cm, respectively. Twelve (52.2%) patients were diagnosed with intrahepatic macro-vascular invasion, and 20 (87.0%) patients had extrahepatic metastasis. The main etiology was hepatitis virus B infection (22/23, 95.7%), and all patients received antiviral therapy before radiotherapy. Fifteen patients had prior treatment, including hepatectomy, transhepatic arterial chemoembolization, chemotherapy, radiofrequency ablation or small molecule tyrosine kinase inhibitors. Eight patients received radiotherapy combined with pembrolizumab and bevacizumab as first-line treatment.
Table 1
| Characteristic | Statistic results (n=23) |
|---|---|
| Age, year, median [range] | 48 [24–67] |
| Gender, n (%) | |
| Female | 1 (4.3) |
| Male | 22 (95.7) |
| ECOG score, n (%) | |
| 0 | 14 (60.9) |
| 1 | 9 (39.1) |
| Maximum tumor size (cm) | |
| Median [range] | 9.7 [3.1–15.0] |
| Mean (95% CI) | 8.6 (5.2–12.1) |
| <5.0, n (%) | 7 (30.4) |
| ≥5.0, n (%) | 16 (69.6) |
| Number of nodules | |
| Median [range] | 3 [1–3] |
| <3, n (%) | 10 (43.5) |
| ≥3, n (%) | 13 (56.5) |
| BCLC staging, n (%) | |
| B | 1 (4.3) |
| C | 22 (95.7) |
| Serum AFP level, n (%) | |
| <400 ng/mL | 11 (47.8) |
| ≥400 ng/mL | 12 (52.2) |
| Cirrhosis, n (%) | 21 (91.3) |
| Hepatitis virus infection, n (%) | |
| HBV | 22 (95.7) |
| HCV | 2 (8.7) |
| HDV | 1 (4.3) |
| Macro-vascular invasion, n (%) | 12 (52.2) |
| Portal vein | 12 (52.2) |
| Hepatic vein | 6 (26.1) |
| Inferior vena cava | 5 (21.7) |
| Hepatic artery | 1 (4.3) |
| Extrahepatic metastasis, n (%) | 20 (87.0) |
| Lymph node | 13 (56.5) |
| Bone | 6 (26.1) |
| Lung | 8 (34.8) |
| Peritoneum | 3 (13.0) |
| Adrenal gland | 2 (8.7) |
| Kidney | 1 (4.3) |
| Cycles of pembrolizumab, median [range] | 4 [1–8] |
| Cycles of bevacizumab, median [range] | 4 [1–9] |
| Radiotherapy dose (Gray) | |
| Median [range] | 45.0 [36.0–48.0] |
| Mean (95% CI) | 42.9 (40.1–45.8) |
| Prior treatment, n (%) | |
| No | 8 (34.8) |
| Hepatectomy | 7 (30.4) |
| Radiofrequency ablation | 3 (13.0) |
| TACE | 9 (39.1) |
| Target therapy | 6 (26.1) |
| Chemotherapy | 1 (4.3) |
ECOG, Eastern Cooperative Oncology Group; CI, confidence interval; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; HBV, hepatitis virus B; HCV, hepatitis virus C; HDV, hepatitis virus D; TACE, transhepatic arterial chemoembolization.
A total of 100 cycles of pembrolizumab were given to 23 patients, and the median was 4 (range, 1–8) cycles. Totally, 91 cycles of bevacizumab were administered to all patients, and the median was 4 (range, 1–9) cycles. All patients received moderately hypo-fractionated IMRT to intrahepatic tumor. The median and mean dose of irradiation was 45.0 (range, 36.0–48.0) Gy and 42.9 (95% CI, 40.1–45.8) Gy, respectively.
Efficacy
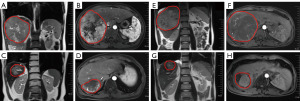
Median follow-up duration was 15.8 (range: 5.4–43.5) months. According to RECIST v1.1 criteria, no patients achieved complete response (CR) (Figure 2 and Table 2). There were 8 partial response (PR), 13 stable disease (SD) and 2 progressive disease (PD) when irradiated intrahepatic tumor was assessed. There were 2 PR, 12 SD and 6 PD when non-irradiated extrahepatic tumor was evaluated. The ORRs and DCRs of irradiated intrahepatic tumor and non-irradiated extrahepatic tumor were 34.8% (95% CI, 16.4–57.3%) and 10.0% (95% CI, 1.2–31.7%), 91.3% (95% CI, 72.0–98.9%) and 70.0% (95% CI, 45.7–88.1%), respectively. Patients of No. 15 (before, Figure 3A,3B; after, Figure 3C,3D) and No. 19 (before, Figure 3E,3F; after, Figure 3G,3H) who had locally advanced, unresectable HCC both achieved PR after 3 Gy*15 f IMRT combined with 4 cycles of bevacizumab and 5 cycles of pembrolizumab as first-line treatment, and finally underwent curative surgical resection. Thirteen patients died from tumor progression. The estimated median PFS was 6.6 (95% CI: 4.7–8.5) months, and estimated median OS was 18.3 (95% CI, 8.2–33.6) months (Figure 4). The estimated 12-month PFS and OS rates were 17.5% (95% CI, 7.0–28%) and 60.9% (95% CI, 50.7–71.1%), respectively. Median DORs of irradiated intrahepatic tumor and non-irradiated extrahepatic tumor were 4.6 and 4.2 months, respectively.
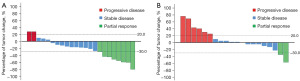
Table 2
| Response | Intrahepatic irradiated tumor | Extrahepatic non-irradiated tumor |
|---|---|---|
| CR, n (%) | 0 | 0 |
| PR, n (%) | 8 (34.8) | 2 (10.0) |
| SD, n (%) | 13 (56.5) | 12 (60.0) |
| PD, n (%) | 2 (8.7) | 6 (30.0) |
| ORR, n (%, 95% CI) | 8 (34.8, 16.4–57.3) | 2 (10.0, 1.2–31.7) |
| DCR, n (%, 95% CI) | 21 (91.3, 72.0–98.9) | 14 (70.0, 45.7–88.1) |
| DOR (months) | 4.6 | 4.2 |
*, according to RECIST v 1.1. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate; DOR, duration of overall response; RECIST, Response Evaluation Criteria in Solid Tumor.

Safety
TRAEs are shown in Table 3. All patients suffered from TRAEs of different grade. The most common TRAEs were anemia (65.2%), thrombocytopenia (52.2%), lymphocytopenia (47.8%), neutropenia (39.1%), leukopenia (34.8%), appetite loss (34.8%), aspartate aminotransferase increase (30.4%) and alanine aminotransferase increase (30.4%), serum bilirubin increase (30.4%), and weight loss (21.7%). Myelosuppression was probably related to radiotherapy. Loss of appetite and weight, and increase of liver enzymes and bilirubin were supposed to result from both radiotherapy and pembrolizumab-bevacizumab. Totally, 18 patients (78.3%) had ≥ grade 3 TRAEs. The most frequent TRAEs of ≥ grade 3 were lymphocytopenia (47.8%), thrombocytopenia (13.0%), aspartate aminotransferase increase (26.1%), alanine aminotransferase increase (13.0%), and leukopenia (13.0%). Decrease of peripheral blood lymphocyte did not affect radiotherapy or systemic treatment. There were no patients who developed classical radiation-induced liver disease or Child-Pugh score progression ≥2. Two patients discontinued radiotherapy for more than one week due to ≥ grade 3 TRAEs (one with grade 3 leukocytopenia, thrombocytopenia, and neutropenia; and the other with grade 3 alanine transaminase increase and gastro-intestinal bleeding). The latter patient also discontinued bevacizumab and pembrolizumab due to bleeding of sigmoid and rectum.
Table 3
| Toxicities | Any grade, n (%) | Grade 3/4, n (%) |
|---|---|---|
| Anemia | 15 (65.2) | 2 (8.7) |
| Thrombocytopenia | 12 (52.2) | 3 (13.0) |
| Lymphocytopenia | 11 (47.8) | 11 (47.8) |
| Neutropenia | 9 (39.1) | 2 (8.7) |
| Leukopenia | 8 (34.8) | 3 (13.0) |
| Appetite loss | 8 (34.8) | 0 |
| Aspartate aminotransferase increase | 7 (30.4) | 6 (26.1) |
| Alanine aminotransferase increase | 7 (30.4) | 3 (13.0) |
| Serum bilirubin increase | 7 (30.4) | 0 |
| Weight loss | 5 (21.7) | 0 |
| Epulis | 2 (8.7) | 2 (8.7) |
| Fatigue | 2 (8.7) | 0 |
| Gastrointestinal hemorrhage | 1 (4.3) | 1 (4.3) |
| Dental ulcer | 1 (4.3) | 1 (4.3) |
| Allergy | 1 (4.3) | 1 (4.3) |
| Rash | 1 (4.3) | 1 (4.3) |
| Nausea | 1 (4.3) | 0 |
*, toxicity grading was according to the US NCI CTCAE v4.03. NCI, National Cancer Institute; CTCAE, Common Terminology Criteria for Adverse Events.
Discussion
This is the first reported study that combined intrahepatic tumor-directed external beam moderately hypo-fractionated radiotherapy, pembrolizumab and bevacizumab for advanced HCC with poor prognosis. This study preliminarily showed that the tri-modality treatment was an effective regimen, without unmanageable or unexpected toxicity.
Former studies indicated that tumor diameter >5 cm, ECOG >0, macroscopic vascular invasion, lymph node involvement, advanced BCLC staging, multiple tumor nodules, extrahepatic invasion or metastasis, viral hepatitis and cirrhosis were adverse prognostic factors for HCC (18-21). In our study, the majority of patients had the inferior prognostic factors mentioned above, especially in aspects of large tumor diameter and high percents of BCLC C stage, macrovascular involvement and extrahepatic metastasis. More than half of patients failed previous treatment. Therefore, treatment for these patients was palliative. The reason of less cycles of pembrolizumab and bevacizumab was partially due to economic burden.
Systemic treatment is recommended for advanced HCC (2). However, efficacy of PD-1/PD-L1 inhibitor or anti-angiogenesis monotherapy was unsatisfactory. The ORR of PD-1/PD-L1 inhibitor monotherapy were 12.4–18.3% (4). ORRs of sorafenib, lenvatinib, regorafenib, ramucirumab, bevacizumab and cabozantinib were 4.0–11.0% (4,22), 18.2–18.8% (23,24), 3.0–10.0% (22,25,26), 7.0–7.3% (27,28), 13.0–14.0% (29,30), and 4.0–4.9% (31,32), respectively. PD-1/PD-L1 inhibitors combined with anti-angiogenesis therapy achieved higher ORRs of 20.5–37%, and improved median PFS of 4.6–9.3 months and OS of 17.1–22.0 months than monotherapy (4). Anti-PD-1/PD-L1 immunotherapy combined with bevacizumab or lenvatinib has been recommended as first-line systemic treatment for advanced HCC. There was growing evidence that intrahepatic tumor-directed local therapy (for example, radiotherapy, trans-arterial chemoembolization, radiofrequency ablation, etc.) alone or combined with target therapy exhibited potential of triggering antitumor immune response (33-35). Radiotherapy is an immune response modifier for immune-oncology. Irradiation to tumor cells leads to immunogenic cell death, releasing DAMPs, and activates cGAS/STING/interferon-I signaling, which promotes the production and release of cytokines and chemokines (36). Interferon-I and DAMPs (such as adenosine triphosphate and high mobility group box 1) stimulate the maturation of dendritic cells and promote the anticancer function of cytotoxic T lymphocytes and natural killer cells (13). Based on the mechanisms mentioned above, radiotherapy might improve the efficacy of immunotherapy plus anti-angiogenesis therapy. A retrospective analysis investigating PD-1/PD-L1 inhibitor plus anti-angiogenesis tyrosine kinase inhibitor plus palliative radiotherapy for advanced HCC reported an ORR of 40%, median PFS of 4.6 months and OS of 21.2 months (15). Recently, START-FIT trial reported that sequential transhepatic arterial chemoembolization and stereotactic body radiation therapy followed by avelumab as conversion therapy for patients with locally advanced, unresectable HCC achieved radiological CR rate of 42%, and 12% of patients had curative surgery (37). Herein, the ORR of irradiated tumor was 34.8% in our cohort, slightly higher than ORR of 20.4% in ORIENT-32 trial investigating an anti-PD-1 monoclonal antibody (sintilimab) plus bevacizumab biosimilar IBI305 (6), and similar to ORR of 27.3% in IMbrave150 trial evaluating atezolizumab plus bevacizumab for advanced HCC (5). The median PFS was 6.6 months, higher than that of 4.6 months in ORIENT-32 trial (6), similar to 6.8 months of PFS in IMbrave150 trial (5). The median OS was 18.3 months, similar to 19.2 months of OS in IMbrave150 trial. The 12-month OS rate was 60.9%, lower than those of 67.2% and approximately 65% in IMbrave150 and ORIENT-32 trials, respectively. When compared to a trial which investigated pembrolizumab plus lenvatinib for unresectable HCC (38), the ORR was similar, but PFS and OS of our cohort were inferior. Explanations for inferior survival here were possibly as follow. (I) Our cohort study had higher percentage of adverse prognostic factors, such as BCLC C stage, macroscopic vascular invasion, extrahepatic metastasis, cirrhosis, and hepatitis virus infection. (II) Our cohort had less treatment duration of pembrolizumab (median, 4 cycles) and bevacizumab (median, 4 cycles) due to unaffordable drug expense. Treatment durations of PD-1/PD-L1 inhibitor plus bevacizumab or lenvatinib in previous studies were more than half year (5,6,38). (III) Radiotherapy was primarily palliative in this study. Discretely distributed metastatic tumor in liver and/or extrahepatic organs could not be included in irradiation target due to dose constraints of organs at risk. For non-irradiated tumor, ORR was only 10%, lower than those of previous studies, possibly due to less duration of systemic treatment; but our DCR was 70%, similar to those of previous studies (5,6). For irradiated tumor, DCR was 91.3%, higher than previous studies (5,6). Therefore, palliative radiotherapy combined with pembrolizumab and bevacizumab predominantly helped to improved local control of tumor. To improve prognosis of advanced HCC, longer duration of anti-PD-1 immunotherapy plus bevacizumab and irradiation to all tumors should be warranted. A single-arm, phase 2, prospective trial reported sequential transhepatic arterial chemoembolization and stereotactic body radiotherapy followed by anti-PD-L1 immunotherapy as conversion therapy for locally advanced unresectable HCC and found that 55% patients were amenable to curative treatment (37). In our study, two patients (2/3) with locally advanced, unresectable HCC achieved PR after 3.0 Gy*15 f IMRT combined with 5 cycles of pembrolizumab and 4 cycles of bevacizumab, and then had curative resection of residual tumors. This trimodal treatment might be a potential conversion therapy for selective locally advanced HCC.
Combining radiotherapy with anti-PD-1/PD-L1 inhibitor and anti-angiogenesis therapy has aroused much interest of clinicians. Regarding to HCC, there are several on-going prospective trials investigating this triple treatment (ChiCTR1900027102, NCT05010434, NCT05096715, NCT04857684, NCT05137899, ChiCTR2200056068). No prospective clinical studies have reported the toxicity profile of this trimodal therapy in advanced HCC. Consistent with results from a retrospective study comparing PD-1 inhibitor plus anti-angiogenic therapy with or without IMRT for advanced HCC (39), in our study adding intrahepatic HCC-directed external beam radiotherapy led to more hepato-toxicities, such as increase of aspartate transaminase, alanine transaminase and serum bilirubin, and more hematologic toxicities, such as anemia, thrombocytopenia, neutropenia and leukopenia. Combining radiotherapy with pembrolizumab and bevacizumab increased incidence of ≥ grade 3 toxicity, which was 78.3% in our study, comparing to 56.0% and 38.0% in ORIENT-32 trial (6) and IMbrave150 trial (5), respectively. The most common ≥ grade 3 toxicities, such as platelet decrease, leukopenia, aspartate transaminase increase and alanine transaminase increase, were probably related to intrahepatic HCC-directed radiotherapy. These could be relieved by thrombopoietin, recombinant human granulocyte stimulating factor, and hepato-protective enzyme-reducing therapy. The spectrums of adverse events observed with intrahepatic HCC radiotherapy plus pembrolizumab plus bevacizumab were consistent with the known toxicity profile of each monotherapy and manageable.
This study is limited by its small sample size and retrospective design. So far, there are no prospective trials reporting efficacy and toxicities of radiotherapy plus pembrolizumab plus bevacizumab for advanced HCC. This real-world study could shed some light on the dosing, sequencing, efficacy and toxicity profile of the tri-modal therapy.
Conclusions
Combining intrahepatic HCC-directed moderately hypo-fractionated radiotherapy with pembrolizumab and bevacizumab was shown to be an effective regimen for advanced HCC. A palliative radiotherapy did not bring unexpected and unmanageable adverse effects. This study offered a potential conversion therapy approach for advanced HCC.
Acknowledgments
Funding: This study was partially supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1333/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1333/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1333/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1333/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Board of the Fifth Affiliated Hospital of Sun Yat-sen University (No. K47-1) and informed consent was taken from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Giannini EG, Bucci L, Garuti F, et al. Patients with advanced hepatocellular carcinoma need a personalized management: A lesson from clinical practice. Hepatology 2018;67:1784-96. [Crossref] [PubMed]
- Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022;19:151-72. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-90. [Crossref] [PubMed]
- Zhu L, Yu X, Wang L, et al. Angiogenesis and immune checkpoint dual blockade in combination with radiotherapy for treatment of solid cancers: opportunities and challenges. Oncogenesis 2021;10:47. [Crossref] [PubMed]
- Schiffmann LM, Brunold M, Liwschitz M, et al. A combination of low-dose bevacizumab and imatinib enhances vascular normalization without inducing extracellular matrix deposition. Br J Cancer 2017;116:600-8. [Crossref] [PubMed]
- Masunaga S, Liu Y, Tanaka H, et al. Reducing intratumour acute hypoxia through bevacizumab treatment, referring to the response of quiescent tumour cells and metastatic potential. Br J Radiol 2011;84:1131-8. [Crossref] [PubMed]
- Chang CC, Dinh TK, Lee YA, et al. Nanoparticle Delivery of MnO(2) and Antiangiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl Mater Interfaces 2020;12:44407-19. [Crossref] [PubMed]
- Lee WS, Yang H, Chon HJ, et al. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020;52:1475-85. [Crossref] [PubMed]
- Siva S, MacManus MP, Martin RF, et al. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 2015;356:82-90. [Crossref] [PubMed]
- De Martino M, Daviaud C, Vanpouille-Box C. Radiotherapy: An immune response modifier for immuno-oncology. Semin Immunol 2021;52:101474. [Crossref] [PubMed]
- Sahebjam S, Forsyth PA, Tran ND, et al. Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: results from a phase I study. Neuro Oncol 2021;23:677-86. [Crossref] [PubMed]
- Zhong L, Wu D, Peng W, et al. Safety of PD-1/PD-L1 Inhibitors Combined with Palliative Radiotherapy and Anti-Angiogenesis Therapy in Advanced Hepatocellular Carcinoma. Front Oncol 2021;11:686621. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-247. [Crossref] [PubMed]
- Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193-202. [Crossref] [PubMed]
- Ding J, Wen Z. Survival improvement and prognosis for hepatocellular carcinoma: analysis of the SEER database. BMC Cancer 2021;21:1157. [Crossref] [PubMed]
- Wörns MA, Bosslet T, Victor A, et al. Prognostic factors and outcomes of patients with hepatocellular carcinoma in non-cirrhotic liver. Scand J Gastroenterol 2012;47:718-28. [Crossref] [PubMed]
- Farinati F, Vitale A, Spolverato G, et al. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med 2016;13:e1002006. [Crossref] [PubMed]
- Gassmann P, Spieker T, Haier J, et al. Prognostic impact of underlying liver fibrosis and cirrhosis after curative resection of hepatocellular carcinoma. World J Surg 2010;34:2442-51. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Choi NR, Kim JY, Hong JH, et al. Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for HBV-associated hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol 2022;22:135. [Crossref] [PubMed]
- Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer 2013;49:3412-9. [Crossref] [PubMed]
- Pelosof L, Lemery S, Casak S, et al. Benefit-Risk Summary of Regorafenib for the Treatment of Patients with Advanced Hepatocellular Carcinoma That Has Progressed on Sorafenib. Oncologist 2018;23:496-500. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Kudo M, Okusaka T, Motomura K, et al. Ramucirumab after prior sorafenib in patients with advanced hepatocellular carcinoma and elevated alpha-fetoprotein: Japanese subgroup analysis of the REACH-2 trial. J Gastroenterol 2020;55:627-39. [Crossref] [PubMed]
- Boige V, Malka D, Bourredjem A, et al. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist 2012;17:1063-72. [Crossref] [PubMed]
- Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 2008;26:2992-8. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Kelley RK, Verslype C, Cohn AL, et al. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol 2017;28:528-34. [Crossref] [PubMed]
- Qi X, Yang M, Ma L, et al. Synergizing sunitinib and radiofrequency ablation to treat hepatocellular cancer by triggering the antitumor immune response. J Immunother Cancer 2020;8:e001038. [Crossref] [PubMed]
- Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer for immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer 2021;9:e003311. [Crossref] [PubMed]
- Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:293-313. [Crossref] [PubMed]
- Vaes RDW, Hendriks LEL, Vooijs M, et al. Biomarkers of Radiotherapy-Induced Immunogenic Cell Death. Cells 2021;10:930. [Crossref] [PubMed]
- Chiang CL, Chiu KWH, Chan KSK, et al. Sequential transarterial chemoembolisation and stereotactic body radiotherapy followed by immunotherapy as conversion therapy for patients with locally advanced, unresectable hepatocellular carcinoma (START-FIT): a single-arm, phase 2 trial. Lancet Gastroenterol Hepatol 2023;8:169-78. [Crossref] [PubMed]
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol 2020;38:2960-70. [Crossref] [PubMed]
- Su K, Guo L, Ma W, et al. PD-1 inhibitors plus anti-angiogenic therapy with or without intensity-modulated radiotherapy for advanced hepatocellular carcinoma: A propensity score matching study. Front Immunol 2022;13:972503. [Crossref] [PubMed]